Continuous Manufacturing
Gericke Formulation Skid GFS
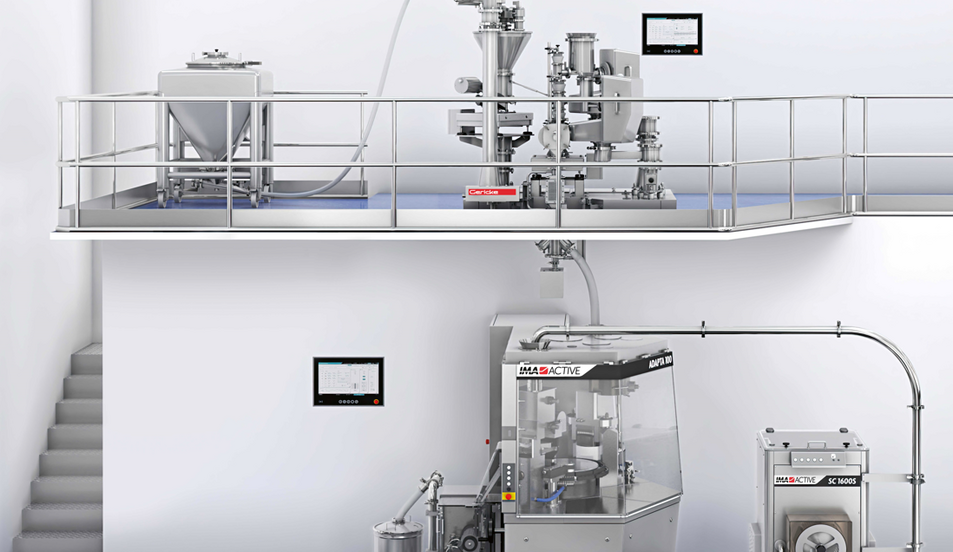
The Gericke Formulation Skid GFS is a fully automated continuous manufacturing method for blending excipients and API upstream compaction, available in two versions: Continuous or Mini-Batch-Blending. It combines high precision loss-in-weight feeders with scientifically designed compact blenders and integrated PAT solutions.
Paradigm shift in pharmaceutical to continuous manufacturing
Most pharmaceutical granules and tablets are produced in batches, not just when preparing the raw components in accordance with the formula but also when mixing the excipients with the active ingredients
After a long period during which the traditional batch process dominated the pharmaceutical manufacturing of OSD, the Food and Drug Administration and the European Medicines recognized the advantages of continuous manufacturing. This removed the regulatory barriers, which historically had held back the increased use of continuous feeding and blending processes in the manufacture of drugs.
The Gericke Formulation Skid can easily be integrated into your existing solid dosage form processes such as:
- Direct Compression
- Wet or Dry Granulation
- Hot Melt Extrusion
- Sachet or Capsule Filling
- As individual unit operation for formulation development

Continuous Feeding and Blending
The Gericke Formulation Skid allows for integration of up to eight loss in weight feeder to a single blender. It can be operated both in truly continuous as well as semi continuous mode for low dose low through put applications.
Advantages of our continuous manufacturing modules
- A Turnkey solution: Skid mounted system including loss in weight feeders, contained refilling equipment and material handling, continuous mixer and PAT solutions for quick and seamless start-up.
- Optimized access to equipment to allow fast cleaning and product changes with minimal down time und high containment.
- Advanced control system ensuring highest product quality and reduced waste.
- Open automation interface to PAT-Management and Advanced Process Controllers.
- Flexibility with downstream processes such as direct compression, wet/dry granulation, or as individual unit operation for formulation development purposes.
- Modular design with interchangeable feeders to suite needs of various processed materials and through put rates.
- Compact mixer for even lowest throughputs with minimal startup losses with easily adaptable residence time and energy input.
Advantages of Continuous Manufacturing
| General: Strengths of continuous processing in the context of mixing solids | Relevance for pharmaceutical solids production |
|---|---|
| Easy to integrate into an existing continuous process | Compression, dry or wet granulation easily become true continuous processes |
| Continuous mixing system takes up less space thanks to the compact dimensions of the equipment | Amount of space required by a feeding/mixing module for 4-6 components is 2*2*2 metres thus drastically reducing valuable GMP space |
| Less risk of demixing, as the process volume is smaller and there is no need to empty large-volume batch mixers or intermediate tanks. Much less material is stored temporarily. | Continuous mixer with a volume of 1-10 litres. Production time required to turn the raw material into a tablet = 5 minutes – "process intensification" |
| Flexible batch size do not require scale up. | Shorter development time, as the same feeding/mixing module is used for the initial test product as for the subsequent marketable product. To increase the "batch size", you extend the production time. |
| The fully automated process cuts operating costs. The manufacturing process and the product quality are assured by means of sophisticated measurement and control technology. | Online measurement and active control of CPPs (Critical Process Parameters) and CQA (Critical Quality Attributes) The continuous feeding and weighing technology represent an integrated P(rocess) A(nalytical) T(echnology) solution that can ensure a formulation accuracy of 0.1 -0.5% thanks to its high-resolution timing and weighing. |
| Improved product quality – the highly homogeneous shear rate and limited residence time distribution mean that the product is subject to very even levels of stress. | Very gentle processing of sensitive products thanks to low speeds and minimal drop heights as well as short mixing times of less than 30 seconds |
Partners for full CM Lines
System integrators:
Equipment vendors:
PAT-Management:
Others:
For more information:
InquiryDownloads
Our customers
Leading Edge Powder Processing Technology